However, for optimal use, this technology must be carried using an efficient delivery system.
An efficient delivery system implies
- A high penetration rate into target cells
- A high level of editing of the targeted gene
- The preservation of the viability and the original cell phenotype
- An all-in-one delivery system (ex: sgRNA guide(s) + Cas9)
With these needs in mind, Vectalys has created a new RNA delivery system named LentiFlash®. Through a fast and transitory RNA expression, this non-integrative particle is particularly suitable to carry CRISPR-Cas9 into any cell type while preserving their original cell phenotype and viability.
The data below, gathered by Vectalys demonstrate that the combination of LentiFlash® with the CRISPR-Cas9 technology can be applied to several therapeutic fields.
LentiFlash® efficiently carries the CRISPR-Cas9 system in an unique particle
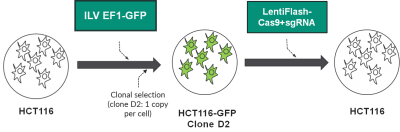
HCT 116 cells have been first stably transduced with an integrative lentiviral vector expressing the GFP reporter. Then, a clone expressing only one copy of the GFP gene has been selected for further use (Clone D2).
This model is used to evaluate improvments of the LentiFlash particle carrying the CRISPR-Cas 9 system. Clone D2 is transduced with Lentiflash carrying the Cas9 and a sgRNA targetting the GFP and the GFP expression level is analyzed.
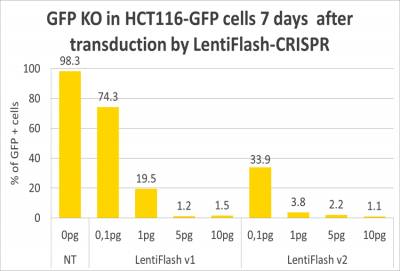
The bar chart below shows the efficiency of LentiFlash-CRISPR-Cas9 in editing GFP in clone D2 cells. With the first LentiFlash generation (v1) 95% of KO is reached with a dose of 5 pg p24 per cell. When using the new LentiFlash generation (v2) the same KO rate (>85%) is achieved using 5 times less particles (1 pg p24 per cell).
Figure 1: Efficient disruption of GFP in clone D2 hct 116 cells using two subsequent LentiFlash generations (data gathered by Vectalys).
LentiFlash® carrying CRISPR-Cas9 for cancer immunotherapy
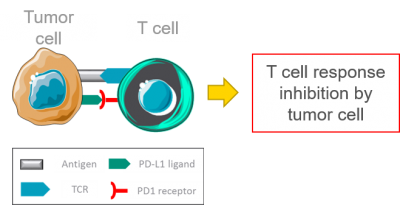
PD-L1 is a ligand expressed at the surface of tumor cells. Its interaction with PD1, its receptor at the surface of T cells, leads to the inhibition of T cells activation. Tumor cells thus evade the immune response (illustration adapted from Servier Medical Art).
The blockade of PD1/PD-L1 interaction has shown promising results in cancer immunotherapy, overcoming the tumor cells escape to T cells immune response.
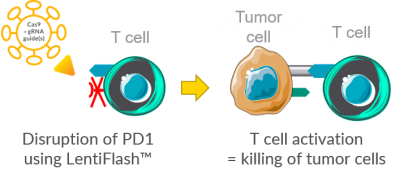
Based on these data, Vectalys has used the LentiFlash delivery system to carry CRISPR-Cas9 into human primary T cells and efficiently disrupt PD1 (illustration adapted from Servier Medical Art).
The bar chart below shows the high efficiency of LentiFlash-CRISPR-Cas9 in editing PD1 in human primary T cells. Indeed, a PD1 disruption rate of 74.8% is reached with a dose of 5 pg p24 per cell. Thanks to the knock-out of PD1, T cells will not be able to interact with PD-L1 and will be effective against the tumor. Therefore, this strategy is a promising approach for future immunotherapeutic programs.

Figure 2: highly efficient disruption of PD1 in human primary T-cells (data gathered by Vectalys)
CAR T cells strategies require to knock-down the endogenous T cell receptor (TCR) in order to express a chimeric receptor (CAR) targeting tumoral cells. The bar chart in figure 3 shows the high efficiency of LentiFlash-CRISPR-Cas9 in editing the alpha chain of the TCR (TRAC gene) in human primary T cells. Indeed, a TCR disruption rate of 72.1% is reached with a dose of 0.1 pg p24 per cell only. Thanks to the knock-out of the TCR, T cells can be subsequently modified with an integrative lentiviral vector to express a CAR of interest at their surface.

Figure 3: Highly efficient disruption of TRAC in human primary T cells (data gathered by Vectalys)
A safe cell engineering
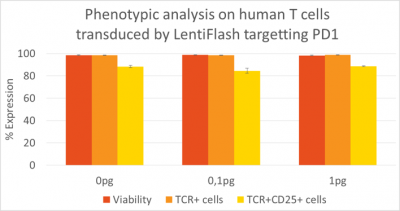
By using highly purified and concentrated LentiFlash particles, human primary T lymphocytes are efficiently transduced without affecting viability and the original cell phenotype is preserved (data gathered by Vectalys).
Figure 4: Preservation of the viability and the original human primary T cell phenotype.
Material & methods
1. Activated T cells are transduced by a range of LentiFlash particles (from 0 to 5 pg of p24/cell) carrying Cas9 and a sgRNA targeting either the human PD1 or the human TRAC gene.
2. Transduction is performed once
3. PD1 or TCR expression is analyzed by FACS, 6 days after transduction, as well as the viability and expression of TCR (for PD1 edited cells) and CD25 on the cells.
The CRISPR approach using LentiFlash RNA delivery leads to a highly efficient and dose-dependent gene knock-out into human T lymphocytes, without any genomic integration.
- Hao Yin, Chun-Qing Song, Joseph R Dorkin, Lihua J Zhu, Yingxiang Li, Qiongqiong Wu, Angela Park, Junghoon Yang, Sneha Suresh, Aizhan Bizhanova, Ankit Gupta, Mehmet F Bolukbasi, Stephen Walsh, Roman L Bogorad, Guangping Gao, Zhiping Weng, Yizhou Dong, Victor Koteliansky, Scot A Wolfe, Robert Langer, Wen Xue, Daniel G Anderson. Therapeutic genome editing by combined viral and non-viral delivery of CRISPR system components in vivo. Nat Biotechnol. Author manuscript; available in PMC 2017 May 9. Published in final edited form as: Nat Biotechnol. 2016 Mar; 34(3): 328–333. Published online 2016 Feb 1. doi: 10.1038/nbt.3471
- Yin H, Kauffman KJ, Anderson DG. Delivery technologies for genome editing. Nat Rev Drug Discov. 2017 Jun;16(6):387-399. Epub 2017 Mar 24. Review. doi: 10.1038/nrd.2016.280.
- Laurence Zitvogel, Guido Kroemer. Targeting PD-1/PD-L1 interactions for cancer immunotherapy. Oncoimmunology. 2012 Nov 1; 1(8): 1223–1225. doi: 10.4161/onci.21335.
-
What is LentiFlash ?
Vectalys
Apply for download
-
Efficient KO of PD1 and CXCR4 in primary T cells using LentiFlash particle expressing CRISPR/Cas9 system (Poster ASGCT 2017)
Vectalys
Apply for download
-
Knock-In and Knock-Out with LentiFlash (Poster ASGCT 2018)
Vectalys
Apply for download
-
Knock-in and knock-out with LentiFlash (Poster ESGCT 2018)
Vectalys
Apply for download
-
All-in-one Delivery Using LentiFlash® technology, an innovative RNA delivery tool for clinical applications (Poster ASGCT 2019)
Vectalys
Apply for download